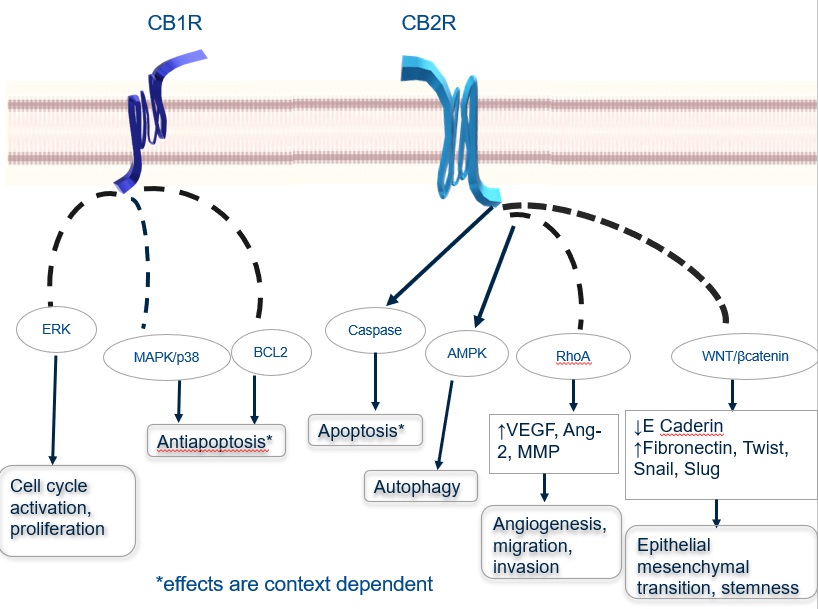
Scholarly Activity and Research covers a wide range of research types. Some examples include quantitative research, qualitative research, experimental research, and case studies. Each type of research has its own unique characteristics and methodology. Understanding these differences can help researchers choose the best approach for their specific needs.